Freezing Point Of Water A. C B. F C. K
pythondeals
Nov 26, 2025 · 11 min read
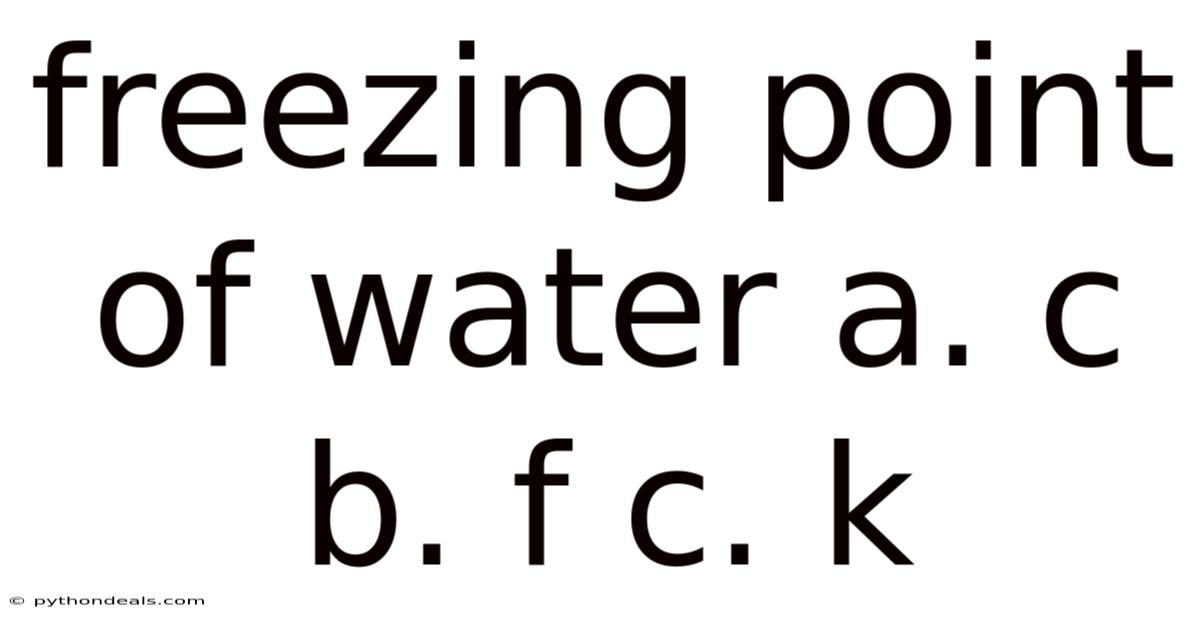
Table of Contents
The seemingly simple question of the freezing point of water unlocks a gateway to understanding fundamental scientific principles, temperature scales, and the unique properties of one of the most vital substances on Earth. Water's transformation from a liquid to a solid, ice, is a cornerstone of our planet's climate, ecosystems, and even our daily lives. This article delves into the intricacies of water's freezing point, exploring its representation across different temperature scales – Celsius, Fahrenheit, and Kelvin – and shedding light on the scientific phenomena that govern this critical transition.
Understanding the freezing point of water requires us to first grasp the concept of temperature itself. Temperature is a measure of the average kinetic energy of the particles within a substance. The faster these particles move, the higher the temperature. When water cools, the kinetic energy of its molecules decreases, causing them to slow down. At a certain point, these molecules lose enough energy that the attractive forces between them become dominant, causing them to arrange themselves into a crystalline structure – ice. This transition occurs at a specific temperature, which we define as the freezing point.
Freezing Point of Water: A Comprehensive Overview
The freezing point of water is defined as the temperature at which water transitions from a liquid state to a solid state (ice). This phase change is a critical parameter in various scientific fields, including chemistry, physics, and environmental science. Water's unique properties, stemming from its molecular structure and hydrogen bonding, heavily influence its freezing point.
- Definition: The freezing point is the temperature at which a liquid transforms into a solid. For pure water at standard atmospheric pressure, this temperature is precisely defined.
- Molecular Behavior: As water cools, the water molecules lose kinetic energy and slow down. At the freezing point, the intermolecular forces, particularly hydrogen bonds, become strong enough to hold the molecules in a fixed crystalline lattice structure, forming ice.
- Standard Conditions: The freezing point is typically referenced under standard atmospheric pressure (1 atmosphere or 101.325 kPa). Changes in pressure can slightly alter the freezing point, a phenomenon described by the Clausius-Clapeyron equation.
- Purity Influence: The presence of impurities in water can lower its freezing point, a phenomenon known as freezing point depression. This principle is utilized in applications such as road de-icing with salt.
Exploring Temperature Scales: Celsius, Fahrenheit, and Kelvin
To accurately represent the freezing point of water, we rely on standardized temperature scales. The three most common scales are Celsius (°C), Fahrenheit (°F), and Kelvin (K). Each scale has its own reference points and unit intervals, making it crucial to understand their relationship when discussing the freezing point of water.
Celsius (°C)
The Celsius scale, also known as the centigrade scale, is part of the metric system and is widely used in scientific and everyday contexts around the world. It's defined by two fixed points:
- 0 °C: The freezing point of pure water at standard atmospheric pressure.
- 100 °C: The boiling point of pure water at standard atmospheric pressure.
The interval between these two points is divided into 100 equal degrees, hence the name "centigrade" (centi- meaning hundred, and grade meaning division). This makes the Celsius scale intuitive for understanding temperature changes relative to water's phase transitions.
Fahrenheit (°F)
The Fahrenheit scale is primarily used in the United States and a few other countries. Its reference points are based on a different system than Celsius:
- 32 °F: The freezing point of pure water at standard atmospheric pressure.
- 212 °F: The boiling point of pure water at standard atmospheric pressure.
The interval between these two points is divided into 180 equal degrees. The Fahrenheit scale's origins are based on a brine solution, which explains why its freezing point for pure water is not at zero.
Kelvin (K)
The Kelvin scale is the absolute thermodynamic temperature scale and is the standard unit of temperature in scientific measurements. It's based on the concept of absolute zero, the theoretical temperature at which all atomic and molecular motion ceases.
- 0 K: Absolute zero, equivalent to -273.15 °C.
- 273.15 K: The freezing point of pure water at standard atmospheric pressure.
- 373.15 K: The boiling point of pure water at standard atmospheric pressure.
The Kelvin scale uses the same degree interval as the Celsius scale, meaning a change of 1 degree Celsius is equivalent to a change of 1 Kelvin. This makes conversions between Celsius and Kelvin straightforward.
The Freezing Point of Water in Different Scales
Now that we've explored the different temperature scales, let's explicitly state the freezing point of water in each:
- Celsius (°C): 0 °C
- Fahrenheit (°F): 32 °F
- Kelvin (K): 273.15 K
These values are crucial for accurate scientific measurements and calculations involving water and its phase transitions.
The Science Behind Freezing: Hydrogen Bonding and Molecular Structure
Water's unique freezing behavior is deeply rooted in its molecular structure and the nature of hydrogen bonding. Water molecules (H₂O) consist of two hydrogen atoms and one oxygen atom arranged in a bent shape. The oxygen atom is more electronegative than the hydrogen atoms, meaning it attracts electrons more strongly, resulting in a partial negative charge on the oxygen and partial positive charges on the hydrogen atoms. This polarity leads to the formation of hydrogen bonds between water molecules.
Hydrogen Bonding:
Hydrogen bonds are relatively weak intermolecular forces that occur between a hydrogen atom with a partial positive charge and a highly electronegative atom (such as oxygen) with a partial negative charge. In liquid water, hydrogen bonds are constantly forming and breaking, allowing the molecules to move relatively freely.
Freezing Process:
As water cools, the kinetic energy of the molecules decreases, and the hydrogen bonds become more stable and organized. At the freezing point, the hydrogen bonds become strong enough to lock the water molecules into a specific crystalline structure – ice. This structure is characterized by a tetrahedral arrangement around each oxygen atom, with each water molecule hydrogen-bonded to four neighboring water molecules.
Anomalous Expansion:
One of the most remarkable properties of water is that it expands when it freezes. This is due to the specific arrangement of molecules in the ice crystal lattice. The tetrahedral arrangement of hydrogen bonds creates a relatively open structure with more space between the molecules than in liquid water. This expansion is why ice floats on water, a crucial factor for aquatic life as it prevents bodies of water from freezing solid from the bottom up.
Factors Affecting the Freezing Point of Water
While we often talk about the freezing point of water as a fixed value, several factors can influence it:
- Pressure: Increasing pressure generally lowers the freezing point of water, although the effect is relatively small. This is because the liquid phase is denser than the solid phase (ice), and increasing pressure favors the denser phase.
- Impurities: The presence of impurities in water lowers its freezing point. This phenomenon is called freezing point depression and is a colligative property, meaning it depends on the concentration of solute particles rather than their identity.
- Salts: Salts, such as sodium chloride (NaCl), are commonly used to de-ice roads because they dissolve in water and lower its freezing point, preventing ice from forming at temperatures below 0 °C (32 °F).
- Sugars: Sugars also lower the freezing point of water, which is why adding sugar to ice cream mix helps prevent large ice crystals from forming, resulting in a smoother texture.
- Supercooling: Under certain conditions, water can be cooled below its freezing point without solidifying. This phenomenon is called supercooling or undercooling. It occurs when the water is very pure and lacks nucleation sites (surfaces or particles that can initiate ice crystal formation). Supercooled water is in a metastable state and will rapidly freeze if disturbed or if a nucleation site is introduced.
Real-World Applications of Understanding Water's Freezing Point
The knowledge of water's freezing point and the factors that affect it has numerous practical applications:
- Weather Forecasting: Accurate weather forecasts rely on understanding the freezing point of water to predict the formation of ice, snow, and other frozen precipitation.
- Agriculture: Farmers need to know the freezing point of water to protect crops from frost damage. Methods like irrigation and covering crops can prevent water from freezing and damaging plant tissues.
- Road Safety: As mentioned earlier, salts are used to de-ice roads and prevent accidents caused by icy conditions. Understanding freezing point depression is crucial for determining the appropriate amount of salt to use.
- Food Preservation: Freezing is a common method of food preservation. Understanding the freezing point of water is essential for optimizing freezing processes and preventing damage to food textures and flavors.
- Cryopreservation: Cryopreservation involves preserving biological materials, such as cells and tissues, at extremely low temperatures. Understanding the freezing point of water is critical for developing effective cryopreservation techniques that minimize ice crystal formation and preserve the viability of the materials.
- Scientific Research: The freezing point of water is a fundamental parameter in many scientific experiments and calculations, particularly in chemistry, physics, and materials science.
Tren & Perkembangan Terbaru
Recent research has focused on understanding the nanoscale behavior of water during freezing. Scientists are using advanced techniques like cryo-electron microscopy and molecular dynamics simulations to study the initial stages of ice nucleation and the structure of ice at the molecular level. These studies are revealing new insights into the role of interfaces, impurities, and confinement on the freezing process.
Another area of active research is the development of new antifreeze materials. Researchers are exploring the use of biomimetic materials inspired by antifreeze proteins found in organisms that survive in freezing environments. These materials could have applications in various fields, including cryopreservation, de-icing, and cold storage.
Furthermore, the impact of climate change on the freezing point of water is a growing concern. Rising global temperatures are causing glaciers and ice sheets to melt at an accelerated rate, contributing to sea-level rise and disrupting ecosystems. Understanding the effects of temperature changes on ice dynamics is crucial for predicting future climate scenarios and developing mitigation strategies.
Tips & Expert Advice
Here are some practical tips and expert advice related to understanding and working with water's freezing point:
- Use a calibrated thermometer: When measuring the freezing point of water in the lab, always use a calibrated thermometer to ensure accurate readings.
- Ensure purity: Use distilled or deionized water to minimize the effects of impurities on the freezing point.
- Control cooling rate: To avoid supercooling, cool the water slowly and provide nucleation sites (e.g., a small ice crystal) to initiate freezing.
- Consider pressure effects: If working at non-standard pressures, be aware that the freezing point may be slightly different. Consult appropriate phase diagrams for accurate values.
- Utilize freezing point depression calculations: When adding solutes to water, use freezing point depression equations to calculate the expected change in freezing point.
- Be mindful of supercooling in practical applications: In applications like ice cream making, be aware of the potential for supercooling and use techniques to promote uniform freezing and prevent large ice crystal formation.
FAQ (Frequently Asked Questions)
- Q: Does salt make water freeze faster?
- A: No, salt lowers the freezing point of water, meaning it needs to be colder for the water to freeze. It doesn't make the water freeze faster at a given temperature.
- Q: Why does ice float?
- A: Ice is less dense than liquid water due to the crystalline structure formed by hydrogen bonds, which creates more space between molecules.
- Q: What is absolute zero?
- A: Absolute zero is the theoretical temperature at which all atomic and molecular motion ceases. It is 0 Kelvin, equivalent to -273.15 °C.
- Q: Can you supercool other liquids besides water?
- A: Yes, supercooling is a general phenomenon that can occur with many liquids under the right conditions.
- Q: How does pressure affect the freezing point of water?
- A: Increasing pressure generally lowers the freezing point of water slightly.
Conclusion
The freezing point of water, whether expressed in Celsius, Fahrenheit, or Kelvin, is more than just a numerical value; it's a gateway to understanding the intricate properties of water and its fundamental role in our world. From the behavior of molecules to the impact of impurities and pressure, and its practical applications in various fields, grasping the science behind water's freezing point is essential. As research continues to unravel the complexities of water at the nanoscale and as climate change alters our planet's temperature dynamics, a deep understanding of this critical parameter will become even more crucial.
How do you think our understanding of water's freezing point can further contribute to innovations in fields like cryopreservation or climate modeling?
Latest Posts
Latest Posts
-
2 1 2 Pints Is How Many Cups
Nov 26, 2025
-
Difference Between A Pond And A Lake
Nov 26, 2025
-
Which Chemical Bond Is The Strongest
Nov 26, 2025
-
Freezing Point Of Water A C B F C K
Nov 26, 2025
-
Currents Shape Rivers Over Long Periods Of Time
Nov 26, 2025
Related Post
Thank you for visiting our website which covers about Freezing Point Of Water A. C B. F C. K . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.