What Are The Spectator Ions In This Equation
pythondeals
Nov 04, 2025 · 10 min read
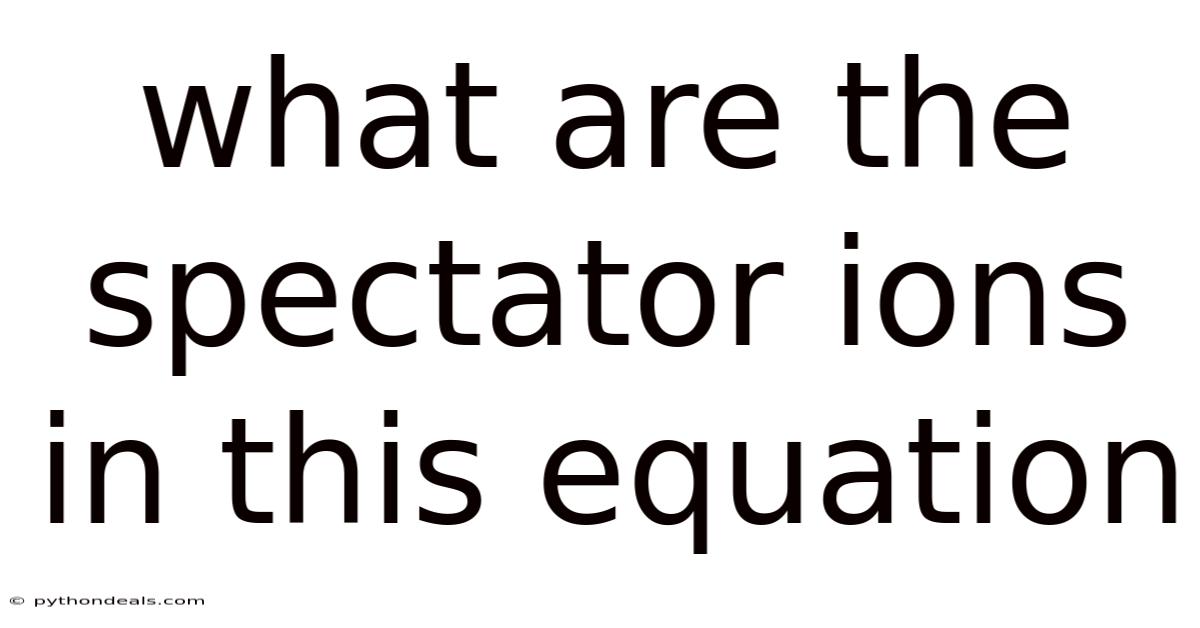
Table of Contents
Okay, let's craft a comprehensive article about spectator ions, ensuring it's informative, engaging, and optimized for readability and SEO.
Understanding Spectator Ions: A Complete Guide
Have you ever looked at a chemical equation and felt like some of the players weren't really doing anything? They're just... there. In the world of chemistry, these seemingly passive participants are often ions, and more specifically, spectator ions. Just as the name implies, they 'spectate' the chemical reaction, observing the process without directly changing themselves.
Spectator ions are crucial for understanding what's actually happening in a reaction, helping us simplify complex equations to focus on the essential interactions. This article will dive deep into the world of spectator ions: what they are, how to identify them, and why they're so important in chemistry.
What are Spectator Ions?
Spectator ions are ions that exist in the same form on both the reactant and product sides of a chemical equation. They're present in the solution, but they do not participate directly in the reaction itself. Imagine them as observers at a sporting event – they're in the stadium (the reaction mixture), but they're not on the field (actively transforming into something new).
Let's break down this definition further:
- Ions: An ion is an atom or molecule that has gained or lost electrons, giving it an electrical charge. Ions with a positive charge are called cations, and ions with a negative charge are called anions.
- Reactant Side: This refers to the substances that start the reaction, located on the left side of the chemical equation.
- Product Side: This refers to the substances that are formed by the reaction, located on the right side of the chemical equation.
- Same Form: This is the key characteristic of spectator ions. They exist as the same ion, with the same charge, on both sides of the equation. They don't combine with other ions, change their charge, or otherwise undergo any chemical transformation.
- Do Not Participate: Spectator ions are not involved in the actual chemical change. They remain dissolved in the solution throughout the reaction, essentially unchanged.
Why are Spectator Ions Important?
You might wonder, if spectator ions don't participate in the reaction, why do we even bother including them in the chemical equation? There are several reasons why understanding spectator ions is vital:
- Representing Realistic Solutions: Chemical reactions often occur in solutions, particularly aqueous (water-based) solutions. Many ionic compounds dissolve in water, dissociating into their constituent ions. Spectator ions are present because they are part of these dissolved ionic compounds. Simply put, these ions contribute to the complete nature of the solution and how the reaction proceeds in a chemical laboratory.
- Balancing Charge: Spectator ions help to maintain charge neutrality in the solution. While they themselves don't react, their presence ensures that the overall charge on both sides of the equation remains balanced.
- Simplifying Equations: Identifying and removing spectator ions allows us to write the net ionic equation, which focuses only on the species that are actively involved in the reaction. This simplifies the equation and highlights the core chemical change.
- Understanding Reaction Mechanisms: In some cases, while spectator ions don't directly react, they can influence the reaction environment (e.g., ionic strength, pH) and therefore, indirectly affect the reaction rate or mechanism.
- Quantitative Analysis: In analytical chemistry, understanding spectator ions is crucial for accurate calculations in titrations, gravimetric analysis, and other quantitative methods. It helps us to differentiate between the ions that contribute to the measured signal and those that do not.
Identifying Spectator Ions: Step-by-Step Guide
Now that we know what spectator ions are and why they're important, let's look at how to identify them in a chemical equation. Here's a step-by-step guide:
-
Write the Balanced Chemical Equation: Make sure the equation is balanced, meaning that the number of atoms of each element and the total charge are the same on both sides of the equation.
- Example: AgNO3(aq) + NaCl(aq) → AgCl(s) + NaNO3(aq)
-
Write the Complete Ionic Equation: This involves breaking down all aqueous ionic compounds into their constituent ions. Remember that solid, liquid, and gaseous compounds do not break apart into ions.
- Example: Ag+(aq) + NO3-(aq) + Na+(aq) + Cl-(aq) → AgCl(s) + Na+(aq) + NO3-(aq)
-
Identify Spectator Ions: Look for ions that appear in the same form on both the reactant and product sides of the complete ionic equation. These are your spectator ions!
- In our example, Na+(aq) and NO3-(aq) are present on both sides.
-
Write the Net Ionic Equation: Remove the spectator ions from the complete ionic equation. This leaves you with the net ionic equation, which shows only the species that are actively involved in the reaction.
- Example: Ag+(aq) + Cl-(aq) → AgCl(s)
Examples of Identifying Spectator Ions
Let's work through a few more examples to solidify our understanding:
-
Example 1: Reaction of Hydrochloric Acid (HCl) with Sodium Hydroxide (NaOH)
- Balanced Chemical Equation: HCl(aq) + NaOH(aq) → NaCl(aq) + H2O(l)
- Complete Ionic Equation: H+(aq) + Cl-(aq) + Na+(aq) + OH-(aq) → Na+(aq) + Cl-(aq) + H2O(l)
- Spectator Ions: Na+(aq) and Cl-(aq)
- Net Ionic Equation: H+(aq) + OH-(aq) → H2O(l)
This net ionic equation shows the fundamental reaction of an acid (H+) with a base (OH-) to form water.
-
Example 2: Reaction of Lead(II) Nitrate (Pb(NO3)2) with Potassium Iodide (KI)
- Balanced Chemical Equation: Pb(NO3)2(aq) + 2KI(aq) → PbI2(s) + 2KNO3(aq)
- Complete Ionic Equation: Pb2+(aq) + 2NO3-(aq) + 2K+(aq) + 2I-(aq) → PbI2(s) + 2K+(aq) + 2NO3-(aq)
- Spectator Ions: K+(aq) and NO3-(aq)
- Net Ionic Equation: Pb2+(aq) + 2I-(aq) → PbI2(s)
This net ionic equation shows the formation of the precipitate lead(II) iodide.
-
Example 3: Reaction of Copper(II) Sulfate (CuSO4) with Zinc Metal (Zn)
- Balanced Chemical Equation: CuSO4(aq) + Zn(s) → ZnSO4(aq) + Cu(s)
- Complete Ionic Equation: Cu2+(aq) + SO42-(aq) + Zn(s) → Zn2+(aq) + SO42-(aq) + Cu(s)
- Spectator Ion: SO42-(aq)
- Net Ionic Equation: Cu2+(aq) + Zn(s) → Zn2+(aq) + Cu(s)
This net ionic equation shows the displacement of copper ions by zinc metal, a type of single replacement reaction.
Common Mistakes to Avoid
Identifying spectator ions is a relatively straightforward process, but here are a few common mistakes to watch out for:
- Forgetting to Balance the Equation: Always start with a balanced chemical equation. An unbalanced equation will lead to incorrect identification of spectator ions and an incorrect net ionic equation.
- Not Breaking Apart Strong Acids, Strong Bases, and Soluble Salts: Strong acids, strong bases, and soluble salts dissociate completely into ions in aqueous solution. Be sure to break these apart in the complete ionic equation. Use solubility rules to decide which ionic compounds are soluble in water and will form ions.
- Breaking Apart Solids, Liquids, and Gases: Solids, liquids, and gases do not break apart into ions in the complete ionic equation. Keep them in their molecular form. Water is a liquid, and it should not be written as H+ and OH- unless it is part of the actual reaction (as in the acid-base reaction example above).
- Confusing Polyatomic Ions: Remember that polyatomic ions (e.g., SO42-, NO3-) remain intact as a single unit. Do not break them down further into individual atoms.
Tren & Perkembangan Terbaru
While the concept of spectator ions is fundamental and well-established in chemistry, its application is constantly evolving with advancements in related fields. Here are some notable trends and developments:
- Computational Chemistry: Computational methods are increasingly used to model chemical reactions and predict the behavior of ions in solution. These simulations can provide insights into the role of spectator ions in influencing reaction kinetics and thermodynamics, even if they don't directly participate in the reaction. For example, research is being done to understand how "spectator ions" affect the hydration sphere around reacting ions, thus influencing reaction rates.
- Electrochemistry: In electrochemical reactions, the ionic environment plays a crucial role in determining the efficiency and selectivity of the process. Understanding the role of spectator ions in the electrolyte solution is essential for optimizing electrode design and reaction conditions. For instance, different spectator ions can affect the electric double layer at the electrode surface.
- Green Chemistry: Green chemistry principles emphasize the use of environmentally friendly solvents and reagents. In this context, the choice of spectator ions can be important for minimizing waste and reducing the environmental impact of chemical processes. Researchers are exploring the use of "greener" salts that yield less toxic spectator ions.
- Materials Science: In the synthesis of nanomaterials and other advanced materials, the ionic environment can influence the size, shape, and properties of the resulting materials. Controlling the concentration and type of spectator ions can be a valuable tool for tailoring material properties.
These developments highlight the continued relevance of spectator ions in modern chemical research and demonstrate how a fundamental concept can be applied to address new challenges in diverse fields.
Tips & Expert Advice
Here are some practical tips and expert advice to help you master the concept of spectator ions:
- Practice, Practice, Practice: The best way to become comfortable with identifying spectator ions is to work through a variety of examples. Start with simple reactions and gradually move on to more complex ones.
- Memorize Solubility Rules: Knowing the solubility rules is essential for writing complete ionic equations. Create a mnemonic or use flashcards to help you remember them.
- Pay Attention to States of Matter: Always pay close attention to the states of matter (solid, liquid, gas, aqueous) when writing complete ionic equations. This will help you avoid the common mistake of breaking apart solids, liquids, and gases into ions.
- Double-Check Your Work: After writing the net ionic equation, double-check that the equation is balanced in terms of both atoms and charge.
- Relate to Real-World Examples: Think about how spectator ions relate to real-world applications, such as water treatment, environmental chemistry, and industrial processes. This will help you appreciate the practical significance of the concept.
- Use Online Resources: There are many excellent online resources available to help you learn about spectator ions, including interactive simulations, practice quizzes, and video tutorials.
- Work with a Study Group: Discussing the concept of spectator ions with your classmates or a study group can help you identify any areas where you are struggling and reinforce your understanding.
FAQ (Frequently Asked Questions)
-
Q: Are spectator ions always present in aqueous solutions?
- A: Yes, spectator ions are typically present in aqueous solutions where ionic compounds are dissolved.
-
Q: Can a molecule be a spectator ion?
- A: No, spectator ions are specifically ions that do not participate in the reaction. Molecules can be present, but if they remain unchanged, they aren't usually referred to as "spectator" species in the same way.
-
Q: What happens to spectator ions after the reaction?
- A: Spectator ions remain dissolved in the solution after the reaction is complete. They do not form a precipitate, gas, or any other new substance.
-
Q: Is it possible for the same ion to be a spectator ion in one reaction but not in another?
- A: Yes, absolutely. Whether an ion is a spectator ion depends entirely on the specific reaction taking place. If it participates in the reaction, it's not a spectator ion.
-
Q: Why are net ionic equations important?
- A: Net ionic equations simplify chemical equations by focusing on the species that are actively involved in the reaction, providing a clearer picture of the core chemical change.
Conclusion
Spectator ions are an essential concept in chemistry, allowing us to simplify complex chemical equations and focus on the actual chemical changes occurring. By understanding how to identify and remove spectator ions, we can write net ionic equations that provide a clear and concise representation of chemical reactions.
From balancing charges in solutions to influencing reaction environments, spectator ions play a more significant role than their name might suggest. So, next time you encounter a chemical equation, remember to look for the spectator ions and appreciate their subtle but important contribution to the world of chemistry.
How do you feel about your ability to spot spectator ions now? Are you ready to tackle some more complex chemical equations?
Latest Posts
Related Post
Thank you for visiting our website which covers about What Are The Spectator Ions In This Equation . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.