One Atmosphere Of Pressure Is Equal To
pythondeals
Nov 22, 2025 · 10 min read
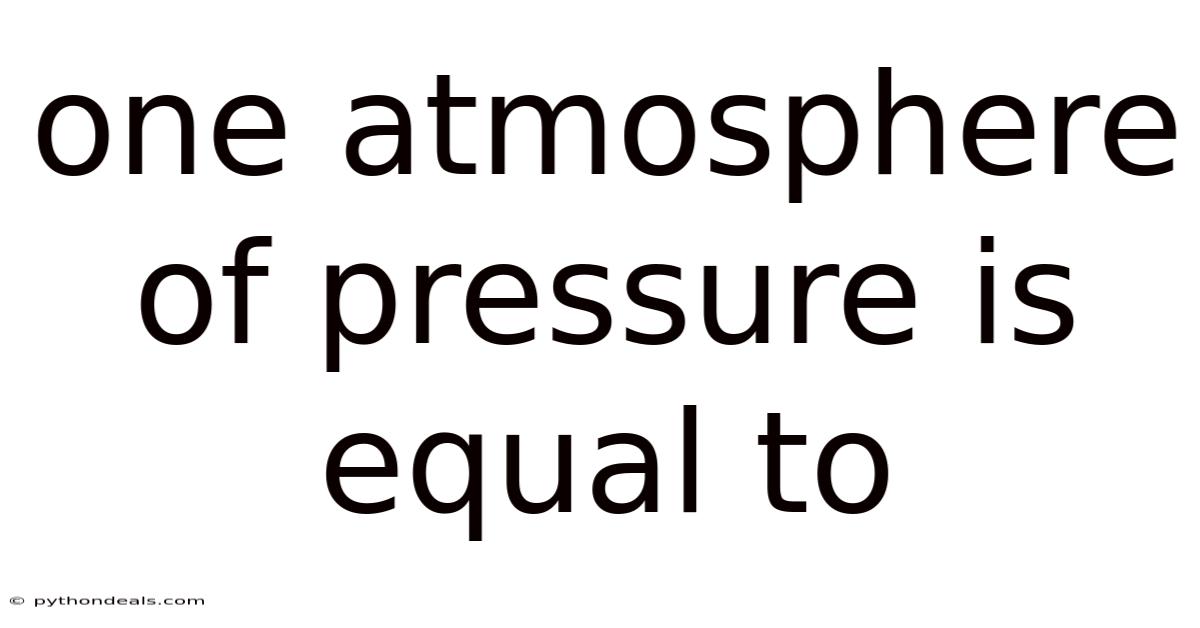
Table of Contents
One Atmosphere of Pressure Is Equal To: A Comprehensive Guide
Have you ever wondered what exactly "one atmosphere of pressure" means? It's a term frequently used in science, engineering, and even everyday life, yet its precise definition and implications can sometimes seem elusive. Understanding atmospheric pressure is crucial for grasping various phenomena, from weather patterns to the behavior of fluids. This comprehensive guide will delve into the concept of one atmosphere of pressure, exploring its origins, its equivalent values in different units, its significance in various fields, and its impact on our daily lives.
Introduction
The concept of "one atmosphere" (atm) is a fundamental unit of pressure. It's essentially a reference point, a standard against which other pressures are measured. Think of it like a baseline – it allows scientists, engineers, and even meteorologists to communicate pressure measurements in a consistent and understandable way. But where does this "atmosphere" come from? It stems from the pressure exerted by the Earth's atmosphere at sea level. Imagine a column of air stretching from the Earth's surface all the way to the edge of space; its weight pressing down on the surface is what defines one atmosphere of pressure. It is important to understand pressure, which is defined as the force applied perpendicular to the surface of an object per unit area over which that force is distributed. Pressure is typically measured in units of pascals (Pa), pounds per square inch (psi), or atmospheres (atm).
This seemingly simple definition, however, opens the door to a deeper understanding of the physical world around us. From the way our lungs function to the design of aircraft, atmospheric pressure plays a vital role. So, let's embark on a journey to unravel the mysteries of "one atmosphere," exploring its various facets and appreciating its profound influence on our lives.
Understanding Pressure: A Deeper Dive
Before we delve into the specifics of one atmosphere, it's crucial to grasp the underlying concept of pressure itself. Pressure, in its simplest form, is the force exerted per unit area. Mathematically, it's expressed as:
Pressure (P) = Force (F) / Area (A)
This formula highlights the key relationship between force, area, and pressure. A larger force applied over a smaller area results in higher pressure. Conversely, the same force applied over a larger area results in lower pressure.
Consider these examples:
- A sharp knife: The sharp edge of a knife has a very small surface area. When you apply force to the knife, this force is concentrated over that tiny area, creating high pressure that allows the knife to easily cut through objects.
- Snowshoes: Snowshoes have a large surface area. By distributing your weight (force) over a larger area, they reduce the pressure exerted on the snow, preventing you from sinking.
- Standing vs. Lying Down: When you stand, your entire weight is concentrated on the relatively small area of your feet. When you lie down, your weight is distributed over a much larger area of your body, resulting in lower pressure on any given point.
The Origin of Atmospheric Pressure
Atmospheric pressure arises from the gravitational force pulling air molecules towards the Earth's surface. Air, although seemingly weightless, has mass. The sheer volume of air above us, combined with gravity, creates a significant downward force, hence pressure.
Several factors influence atmospheric pressure, including:
- Altitude: As you ascend in altitude, the amount of air above you decreases. This means there is less weight pressing down, leading to lower atmospheric pressure. This is why airplanes need pressurized cabins and why climbers experience shortness of breath at high altitudes.
- Temperature: Warmer air is less dense than cooler air. Less dense air exerts less pressure. Therefore, atmospheric pressure tends to be lower in warmer regions and higher in cooler regions.
- Humidity: Humid air is slightly less dense than dry air because water molecules (H₂O) are lighter than the nitrogen and oxygen molecules that make up the majority of the atmosphere. This means humid air exerts slightly less pressure.
- Weather Systems: High-pressure systems are associated with descending air, leading to increased pressure at the surface. These systems typically bring clear skies and stable weather. Low-pressure systems, on the other hand, are associated with rising air, leading to decreased pressure and often bringing cloudy skies and precipitation.
One Atmosphere: Defining the Standard
Now that we have a solid understanding of pressure and atmospheric pressure, let's focus on "one atmosphere." By definition, one atmosphere (1 atm) is the pressure exerted by the Earth's atmosphere at sea level under standard conditions. "Standard conditions" are usually defined as 0 degrees Celsius (273.15 Kelvin) and at sea level.
The precise value of 1 atm has been standardized to ensure consistency in scientific calculations and engineering applications. While the exact value might vary slightly depending on the source and the specific definition of "standard conditions" being used, the following values are generally accepted:
- 1 atm = 101,325 Pascals (Pa)
- 1 atm = 101.325 Kilopascals (kPa)
- 1 atm = 14.696 Pounds per Square Inch (psi)
- 1 atm = 760 Torr
- 1 atm = 760 mmHg (millimeters of mercury)
- 1 atm = 1.01325 bar
It's important to remember these conversions, as they are frequently used in various scientific and engineering contexts. You might encounter pressure values given in any of these units, so being able to convert between them is essential.
The Significance of One Atmosphere in Different Fields
One atmosphere serves as a crucial reference point in numerous scientific and engineering disciplines:
- Meteorology: Meteorologists use atmospheric pressure measurements to predict weather patterns. Changes in atmospheric pressure indicate approaching weather systems. A falling barometer (an instrument used to measure atmospheric pressure) often signals an approaching storm, while a rising barometer indicates improving weather.
- Aviation: Aircraft cabins are pressurized to maintain a comfortable and safe environment for passengers and crew at high altitudes where the external atmospheric pressure is significantly lower. The cabin pressure is typically maintained at an equivalent of about 8,000 feet above sea level, which is still lower than the pressure at sea level, but significantly higher than the outside pressure at cruising altitude.
- Diving: Divers experience increased pressure as they descend underwater. For every 10 meters (approximately 33 feet) of depth, the pressure increases by approximately 1 atmosphere. Understanding pressure is crucial for divers to avoid decompression sickness (the bends), a potentially life-threatening condition caused by rapid changes in pressure.
- Chemistry: Many chemical reactions are affected by pressure. One atmosphere is often used as a standard condition for reporting the results of chemical experiments. The volume of a gas depends on the pressure. Boyle's Law demonstrates the inverse relationship between pressure and volume.
- Engineering: In various engineering applications, such as designing pipelines, pressure vessels, and hydraulic systems, a thorough understanding of pressure and its effects on materials is paramount. Engineers must account for atmospheric pressure, as well as other internal and external pressures, to ensure the safety and integrity of these structures. For example, the pressure vessels used in many industrial processes must be built to withstand the pressure.
- Medicine: Understanding atmospheric pressure is important in medicine. For example, ventilators need to be calibrated to deliver the appropriate pressure to patients. Furthermore, hyperbaric oxygen therapy involves exposing patients to higher than normal pressures to treat certain medical conditions.
- Cooking: In high-altitude cooking, adjustments need to be made to recipes because water boils at a lower temperature due to the reduced atmospheric pressure. This affects cooking times and the consistency of the final product.
- Materials Science: The behavior of materials under pressure is a critical area of study in materials science. High-pressure experiments are used to synthesize new materials with unique properties.
Impact on Daily Life
While we may not always be consciously aware of it, atmospheric pressure and the concept of "one atmosphere" affect our daily lives in numerous ways:
- Breathing: Our bodies are designed to function optimally at atmospheric pressure. The pressure difference between the air in our lungs and the external atmosphere allows us to breathe.
- Weather: As mentioned earlier, atmospheric pressure plays a crucial role in determining weather patterns. By monitoring changes in atmospheric pressure, we can anticipate storms, changes in temperature, and other weather events.
- Food Preservation: Canning food relies on creating a vacuum inside the jar, which reduces the pressure and inhibits the growth of microorganisms, thereby preserving the food.
- Tire Pressure: Maintaining the correct tire pressure in our vehicles is essential for fuel efficiency, tire longevity, and safety. Tire pressure gauges typically measure the pressure relative to atmospheric pressure.
- Packaging: Many food and beverage products are packaged under pressure to maintain freshness and prevent spoilage. Carbonated drinks, for example, are pressurized with carbon dioxide to create the fizz.
Beyond One Atmosphere: Exploring Higher and Lower Pressures
While "one atmosphere" serves as a crucial reference point, it's important to understand that pressures can vary significantly in different environments.
- Higher Pressures: Deep-sea environments experience extremely high pressures. For example, the pressure at the bottom of the Mariana Trench, the deepest point in the ocean, is over 1,000 times greater than atmospheric pressure. Specialized equipment and submersibles are required to explore these extreme environments.
- Lower Pressures: Space, on the other hand, is essentially a vacuum with extremely low pressure. Space suits are necessary to protect astronauts from the harsh environment of space, providing them with a pressurized environment that mimics Earth's atmosphere.
Tips & Expert Advice
- Use a Barometer: Consider purchasing a barometer for your home. Monitoring changes in atmospheric pressure can provide valuable insights into upcoming weather conditions.
- Understand Tire Pressure: Regularly check and maintain the correct tire pressure in your vehicle. Refer to your vehicle's owner's manual for the recommended tire pressure.
- Be Aware of Altitude: If you plan to travel to high altitudes, be aware of the potential effects of lower atmospheric pressure. Drink plenty of water, avoid strenuous activity, and allow your body to acclimatize gradually.
- Practice Unit Conversions: Familiarize yourself with the different units of pressure and practice converting between them. This will be helpful in various scientific and engineering contexts.
- Explore Online Resources: Numerous online resources provide detailed information on atmospheric pressure, pressure conversions, and related topics. Use these resources to expand your knowledge and understanding.
FAQ (Frequently Asked Questions)
- Q: What is the difference between pressure and force?
- A: Force is a push or pull, while pressure is the force applied per unit area.
- Q: Why does atmospheric pressure decrease with altitude?
- A: As altitude increases, the amount of air above you decreases, resulting in less weight pressing down and lower atmospheric pressure.
- Q: What is the standard temperature and pressure (STP)?
- A: STP is defined as 0 degrees Celsius (273.15 Kelvin) and 1 atmosphere of pressure.
- Q: How does humidity affect atmospheric pressure?
- A: Humid air is slightly less dense than dry air, so it exerts slightly less pressure.
- Q: Is atmospheric pressure constant?
- A: No, atmospheric pressure varies with altitude, temperature, humidity, and weather systems.
Conclusion
One atmosphere of pressure, equivalent to 101,325 Pascals, 14.696 psi, or 760 mmHg, is a fundamental unit of pressure derived from the pressure exerted by Earth's atmosphere at sea level. Understanding its significance is crucial in numerous fields, including meteorology, aviation, diving, chemistry, and engineering. From predicting weather patterns to designing safe aircraft cabins, atmospheric pressure plays a vital role in our world. It also affects our daily lives in subtle but significant ways, from the way we breathe to the way we preserve food. By grasping the concept of "one atmosphere," we gain a deeper appreciation for the physical forces that shape our environment.
How does this knowledge influence your understanding of the world around you? Are you inspired to explore the science of atmospheric pressure further?
Latest Posts
Latest Posts
-
How Are Comets And Asteroids Similar
Nov 22, 2025
-
How To Factor X 3 2
Nov 22, 2025
-
Function Of Cytoplasmic Membrane In Bacteria
Nov 22, 2025
-
What Is A Calibration Curve In Chemistry
Nov 22, 2025
-
Do Mushrooms Make Their Own Food
Nov 22, 2025
Related Post
Thank you for visiting our website which covers about One Atmosphere Of Pressure Is Equal To . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.