Is Cooh An Acid Or Base
pythondeals
Nov 11, 2025 · 10 min read
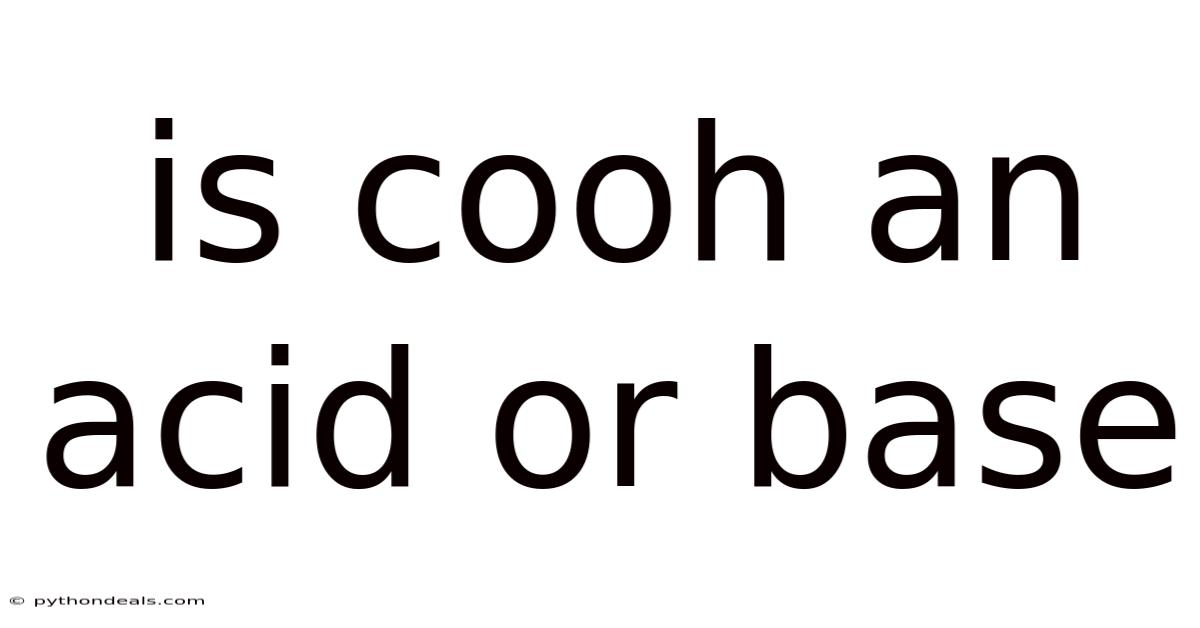
Table of Contents
Let's dive into the chemistry of -COOH, the functional group known as the carboxyl group, and explore whether it acts as an acid or a base. We'll cover the structure, properties, and reactions that dictate its acidic behavior. Understanding the carboxyl group is fundamental to grasping the nature of organic acids and their importance in various chemical and biological processes.
Introduction
The carboxyl group (-COOH) is a ubiquitous functional group in organic chemistry, found in carboxylic acids like acetic acid (vinegar) and fatty acids (components of lipids). These compounds play crucial roles in countless chemical reactions and biological processes. Understanding whether -COOH acts as an acid or a base is essential for comprehending its chemical behavior. In short, the carboxyl group is acidic. The acidity arises from the ability of the -COOH group to donate a proton (H+), forming a carboxylate ion (-COO-). The stability of this carboxylate ion, due to resonance, further enhances the acidity of the carboxyl group.
Let's explore the reasons behind its acidic nature and the factors influencing its acidity.
Comprehensive Overview
To understand the acidic nature of the carboxyl group, we need to delve into its structure, properties, and the underlying principles that govern its behavior.
-
Structure of the Carboxyl Group:
The carboxyl group consists of a carbon atom double-bonded to an oxygen atom (carbonyl group, C=O) and single-bonded to a hydroxyl group (-OH). This combination gives rise to the -COOH designation. The carbon atom is sp2 hybridized, leading to a planar geometry around the carbon atom. The bond angles are approximately 120 degrees.
-
Polarity and Electronegativity:
Oxygen is more electronegative than both carbon and hydrogen. This electronegativity difference results in polar bonds within the carboxyl group. The oxygen atoms pull electron density away from the carbon and hydrogen atoms, creating partial negative charges (δ-) on the oxygen atoms and partial positive charges (δ+) on the carbon and hydrogen atoms. This polarity is crucial for the acidic behavior of the carboxyl group.
-
Acidity of Carboxylic Acids:
Carboxylic acids are Brønsted-Lowry acids, which means they are proton (H+) donors. When a carboxylic acid donates a proton, it forms a carboxylate ion (-COO-).
R-COOH <----> R-COO- + H+The acidity of a carboxylic acid is quantified by its acid dissociation constant (Ka) or its pKa value, where pKa = -log(Ka). A lower pKa value indicates a stronger acid. Carboxylic acids typically have pKa values in the range of 4 to 5, making them weak acids. This means that they do not completely dissociate in water.
-
Resonance Stabilization of the Carboxylate Ion:
The key reason for the acidity of carboxylic acids lies in the stability of the carboxylate ion. The negative charge on the carboxylate ion is delocalized over the two oxygen atoms through resonance. This delocalization spreads the negative charge, making the ion more stable than if the charge were localized on a single oxygen atom.
Resonance structures of the carboxylate ion:
R-C(=O)-O- <----> R-C(-O)-=OThis resonance stabilization lowers the energy of the carboxylate ion, shifting the equilibrium towards the formation of the carboxylate ion and the proton (H+), thus increasing the acidity of the carboxylic acid.
-
Factors Affecting Acidity:
Several factors can influence the acidity of carboxylic acids, including:
- Inductive Effects: Electronegative atoms or groups attached to the carbon chain near the carboxyl group can increase the acidity. These groups pull electron density away from the carboxyl group, further stabilizing the carboxylate ion. For example, chloroacetic acid (ClCH2COOH) is more acidic than acetic acid (CH3COOH) due to the electron-withdrawing effect of the chlorine atom.
- Resonance Effects: The presence of other groups that can participate in resonance can also affect acidity. For example, if a group can donate electron density through resonance, it can destabilize the carboxylate ion and decrease the acidity.
- Solvent Effects: The solvent in which the carboxylic acid is dissolved can also affect its acidity. Protic solvents (such as water and alcohols) can stabilize the carboxylate ion through hydrogen bonding, increasing the acidity. Aprotic solvents (such as dimethyl sulfoxide) do not have this effect.
-
Comparison to Other Acids:
Carboxylic acids are considered weak acids compared to strong mineral acids like hydrochloric acid (HCl) or sulfuric acid (H2SO4). Strong acids completely dissociate in water, while carboxylic acids only partially dissociate. However, carboxylic acids are more acidic than alcohols (ROH) or phenols (ArOH). Alcohols have pKa values around 16-18, and phenols have pKa values around 10. The acidity of phenols is due to resonance stabilization of the phenoxide ion, but it is less effective than the resonance stabilization in carboxylate ions.
-
Examples of Carboxylic Acids:
- Acetic Acid (CH3COOH): Found in vinegar, acetic acid has a pKa of 4.76. It is a weak acid commonly used in various chemical and biological applications.
- Formic Acid (HCOOH): The simplest carboxylic acid, formic acid has a pKa of 3.75. It is found in ant stings and is used in various industrial processes.
- Benzoic Acid (C6H5COOH): An aromatic carboxylic acid, benzoic acid has a pKa of 4.20. It is used as a preservative and in the synthesis of other organic compounds.
- Fatty Acids: Long-chain carboxylic acids, such as stearic acid (C18H36O2) and oleic acid (C18H34O2), are essential components of lipids and cell membranes.
Tren & Perkembangan Terbaru
Recent trends and developments in the study of carboxylic acids focus on their applications in various fields, including drug discovery, materials science, and green chemistry.
- Drug Discovery: Carboxylic acids are common functional groups in drug molecules. Many drugs contain carboxylic acid moieties, which interact with biological targets through hydrogen bonding and other non-covalent interactions. Researchers are exploring new methods for synthesizing carboxylic acid-containing compounds and for modifying existing carboxylic acids to improve their pharmacological properties.
- Materials Science: Carboxylic acids are used as building blocks for the synthesis of polymers and other materials. For example, dicarboxylic acids (containing two carboxyl groups) are used in the production of polyesters and polyamides. Recent research focuses on the development of bio-based carboxylic acids from renewable resources for sustainable materials.
- Green Chemistry: Carboxylic acids are being explored as alternatives to traditional organic solvents and catalysts. They can act as Brønsted acid catalysts in various organic reactions, offering a greener alternative to toxic and corrosive mineral acids. Researchers are also developing new methods for the synthesis of carboxylic acids from renewable feedstocks, such as biomass and CO2.
- Advances in Analytical Techniques: Advanced analytical techniques such as mass spectrometry and nuclear magnetic resonance (NMR) spectroscopy allow for precise analysis and identification of carboxylic acids in complex mixtures. These techniques have become essential tools in environmental monitoring, food analysis, and metabolomics research.
- Carboxylic Acids in Energy Storage: Carboxylic acids and their derivatives are being researched for applications in energy storage devices such as lithium-ion batteries. For example, certain carboxylic acid-containing polymers can act as electrode materials, providing high capacity and improved performance.
- Environmental Applications: Carboxylic acids play a crucial role in environmental chemistry. They are involved in the acidification of rainwater and the formation of acid mine drainage. Understanding the behavior and fate of carboxylic acids in the environment is essential for developing strategies to mitigate pollution and protect ecosystems.
Tips & Expert Advice
As someone deeply involved in chemistry, here are some tips and expert advice to help you better understand the role and handling of carboxylic acids:
- Understand the pKa Scale: A solid understanding of the pKa scale is essential for predicting the behavior of carboxylic acids in different chemical environments. Remember, lower pKa values indicate stronger acids. Use pKa values to compare the acidity of different carboxylic acids and to predict their behavior in reactions.
- Consider Inductive Effects: When comparing the acidity of different carboxylic acids, always consider the inductive effects of substituents. Electron-withdrawing groups near the carboxyl group will increase the acidity, while electron-donating groups will decrease the acidity.
- Resonance is Key: Always remember that the stability of the carboxylate ion due to resonance is the primary reason for the acidity of carboxylic acids. Visualize the resonance structures of the carboxylate ion to understand how the negative charge is delocalized.
- Practical Applications: Carboxylic acids are versatile compounds with numerous applications in chemistry, biology, and industry. Study the specific uses of different carboxylic acids to appreciate their importance in these fields.
- Safety Precautions: When working with carboxylic acids in the laboratory, always follow proper safety precautions. Some carboxylic acids can be corrosive or toxic. Wear appropriate personal protective equipment (PPE), such as gloves, safety goggles, and a lab coat, and work in a well-ventilated area.
- Titration Techniques: Mastering titration techniques is crucial for quantifying the concentration of carboxylic acids in solutions. Titration involves the gradual addition of a base to neutralize the acid, allowing for precise determination of its concentration.
- Spectroscopic Analysis: Learn to interpret spectroscopic data, such as NMR and IR spectra, to identify and characterize carboxylic acids in complex mixtures. Spectroscopic techniques provide valuable information about the structure and properties of carboxylic acids.
- Stay Updated: Keep up with the latest research and developments in the field of carboxylic acid chemistry. New discoveries and applications are constantly emerging, so staying informed is essential for advancing your knowledge and skills.
FAQ (Frequently Asked Questions)
- Q: Are all organic acids carboxylic acids?
- A: No, not all organic acids are carboxylic acids. There are other types of organic acids, such as sulfonic acids and phenols, which contain different acidic functional groups.
- Q: Why are carboxylic acids weak acids?
- A: Carboxylic acids are weak acids because they only partially dissociate in water. The equilibrium between the carboxylic acid and the carboxylate ion favors the undissociated acid.
- Q: How can I increase the acidity of a carboxylic acid?
- A: You can increase the acidity of a carboxylic acid by attaching electron-withdrawing groups near the carboxyl group. These groups stabilize the carboxylate ion, shifting the equilibrium towards dissociation.
- Q: What are some common uses of carboxylic acids?
- A: Carboxylic acids are used in a wide range of applications, including the production of polymers, pharmaceuticals, food preservatives, and cleaning agents.
- Q: Can carboxylic acids act as bases?
- A: While carboxylic acids are primarily known for their acidic properties, they can act as very weak bases under certain conditions. This typically involves the oxygen atoms in the carboxyl group accepting a proton. However, their basicity is much weaker than their acidity.
- Q: How does temperature affect the acidity of carboxylic acids?
- A: Generally, increasing the temperature slightly increases the dissociation of carboxylic acids, leading to a marginal increase in acidity. However, the effect is usually small unless the temperature change is substantial.
Conclusion
In summary, the carboxyl group (-COOH) is indeed acidic due to its ability to donate a proton (H+) and form a resonance-stabilized carboxylate ion (-COO-). This acidic behavior is influenced by factors such as inductive effects, resonance effects, and solvent effects. Carboxylic acids are weak acids compared to strong mineral acids, but they are more acidic than alcohols and phenols. Their versatile properties and wide range of applications make carboxylic acids essential compounds in chemistry, biology, and industry. Understanding the structure, properties, and reactions of the carboxyl group is crucial for comprehending its chemical behavior and its role in various processes.
How do you plan to apply this knowledge in your future studies or projects? Are there specific aspects of carboxylic acid chemistry that you find particularly intriguing or challenging?
Latest Posts
Latest Posts
-
Where Does Pyruvate Go After Glycolysis
Nov 11, 2025
-
What Is A Zeroth Order Reaction
Nov 11, 2025
-
Natural Rights And Declaration Of Independence
Nov 11, 2025
-
How To Use Inverse Matrices To Solve System Of Equations
Nov 11, 2025
-
Which Direction Do Latitude Lines Run
Nov 11, 2025
Related Post
Thank you for visiting our website which covers about Is Cooh An Acid Or Base . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.