Diffusion Is A Process Which Depends On Concentration Gradients.
pythondeals
Nov 08, 2025 · 9 min read
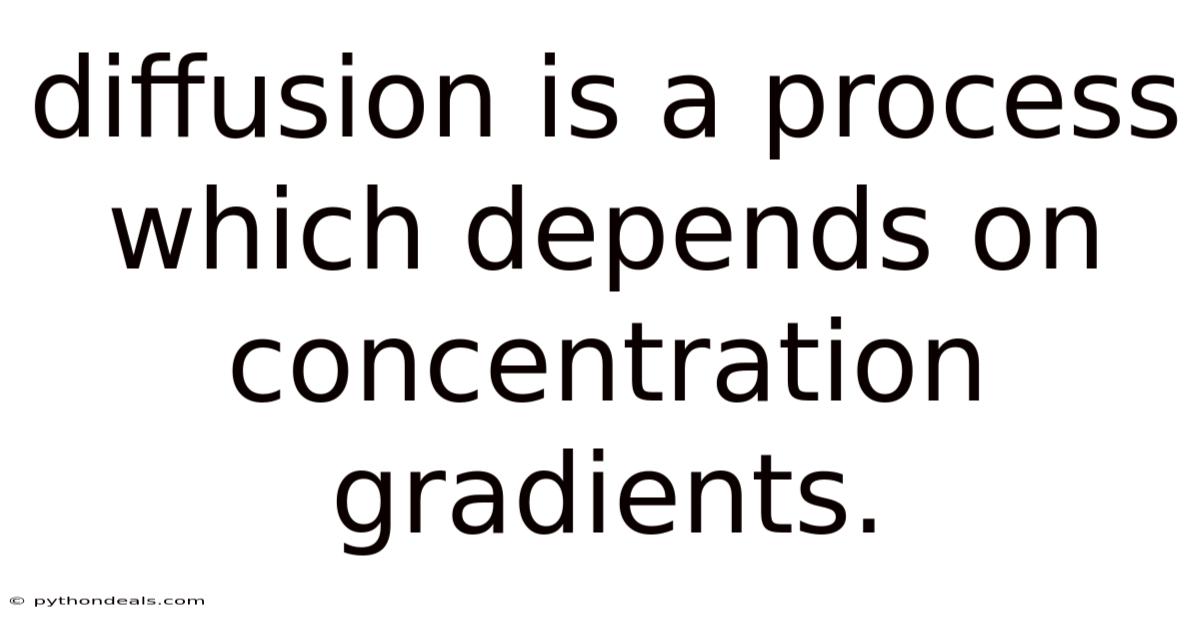
Table of Contents
Diffusion: A Dance Driven by Concentration Gradients
Imagine dropping a single drop of food coloring into a glass of water. Initially, the color is intensely concentrated in one spot. But over time, something magical happens: the color gradually spreads out, eventually coloring the entire glass a uniform hue. This is diffusion in action, a fundamental process that underpins countless phenomena, from the air we breathe to the inner workings of our cells. At its heart, diffusion is a process intrinsically linked to concentration gradients, driven by the relentless quest for equilibrium.
Concentration gradients are the unsung heroes of this story. They represent the difference in concentration of a substance between two regions. Like a hill that a ball naturally rolls down, molecules tend to move from areas of high concentration to areas of low concentration, seeking to minimize this difference. This movement, driven by the inherent thermal energy of molecules, is what we call diffusion. The steeper the concentration gradient, the faster the diffusion occurs, making it a crucial factor in the rate and extent of this ubiquitous process.
Delving Deeper: Understanding the Mechanics of Diffusion
Diffusion, at its core, is the net movement of molecules from a region of higher concentration to a region of lower concentration. This movement is a direct consequence of the random thermal motion of molecules. Even in seemingly still environments, molecules are constantly jiggling, bumping, and colliding with one another. These random movements are what propel diffusion.
Think of it like this: imagine a crowded room on one side of a door and an empty room on the other. When the door opens, people from the crowded room will naturally start to move into the empty room, simply because there are more people on one side and more space on the other. This isn't a coordinated effort; each person is moving randomly, but the overall effect is a movement from high density to low density.
Similarly, in diffusion, individual molecules move randomly, but because there are more molecules in the area of high concentration, there are statistically more molecules moving out of that area than moving into it. This leads to a net movement of molecules down the concentration gradient.
There are two primary types of diffusion to consider:
-
Simple Diffusion: This occurs when molecules move across a membrane or through a space without the aid of any transport proteins or other facilitating mechanisms. The driving force is solely the concentration gradient. Small, nonpolar molecules like oxygen and carbon dioxide can readily diffuse across cell membranes via simple diffusion.
-
Facilitated Diffusion: This type of diffusion requires the assistance of membrane proteins. These proteins bind to specific molecules and facilitate their passage across the membrane. While still driven by the concentration gradient, facilitated diffusion allows larger or polar molecules, like glucose and amino acids, to cross the membrane. There are two types of proteins involved in facilitated diffusion, channel proteins and carrier proteins. Channel proteins create a hydrophilic pore through the membrane, allowing specific molecules to move through. Carrier proteins bind to the molecule being transported, change shape, and release the molecule on the other side of the membrane.
The Central Role of Concentration Gradients
The concentration gradient is the engine that drives diffusion. Without a difference in concentration, there would be no net movement of molecules. The steeper the concentration gradient, the faster the rate of diffusion. This relationship is described by Fick's First Law of Diffusion, which states that the flux (rate of diffusion) is proportional to the concentration gradient.
Mathematically, Fick's First Law can be expressed as:
J = -D (dC/dx)
Where:
- J is the diffusion flux (amount of substance diffusing per unit area per unit time)
- D is the diffusion coefficient (a measure of how easily a substance diffuses through a particular medium)
- dC/dx is the concentration gradient (the change in concentration with respect to distance)
The negative sign indicates that diffusion occurs down the concentration gradient, from high to low concentration.
This equation highlights the direct relationship between the rate of diffusion and the concentration gradient. A larger concentration gradient (dC/dx) will result in a larger diffusion flux (J), assuming the diffusion coefficient (D) remains constant.
Factors Influencing Diffusion Beyond Concentration Gradients
While concentration gradients are the primary driving force behind diffusion, other factors can also influence the rate and extent of this process:
- Temperature: Higher temperatures increase the kinetic energy of molecules, leading to faster movement and, consequently, faster diffusion. The diffusion coefficient (D) in Fick's Law is directly proportional to temperature.
- Molecular Weight: Smaller, lighter molecules tend to diffuse faster than larger, heavier molecules. This is because they have higher velocities at the same temperature.
- Medium Density: Diffusion occurs more slowly in denser media. For example, diffusion is faster in air than in water, and faster in water than in a gel.
- Membrane Permeability: In biological systems, the permeability of a membrane to a particular substance significantly affects the rate of diffusion. Membranes that are highly permeable to a substance will allow it to diffuse more readily.
- Surface Area: A larger surface area allows for a greater number of molecules to diffuse across a boundary in a given time.
Real-World Examples: Diffusion in Action
Diffusion is not just a theoretical concept; it is a fundamental process that plays a vital role in numerous natural and technological phenomena:
- Respiration: In the lungs, oxygen diffuses from the air into the blood, while carbon dioxide diffuses from the blood into the air. This gas exchange is essential for respiration and depends entirely on the concentration gradients of these gases.
- Nutrient Uptake: In the small intestine, nutrients from digested food diffuse across the intestinal lining and into the bloodstream.
- Waste Removal: In the kidneys, waste products from the blood diffuse into the urine.
- Drug Delivery: Many drugs are designed to diffuse across cell membranes to reach their target sites within the body. The rate of diffusion can significantly impact the effectiveness of a drug.
- Air Fresheners: The pleasant scent from an air freshener diffuses throughout the room, filling the space with fragrance.
- Flavoring Food: When you add salt to water, the salt molecules diffuse throughout the water, evenly distributing the flavor.
- Industrial Processes: Diffusion is used in a variety of industrial processes, such as the separation of gases, the purification of liquids, and the manufacturing of semiconductors.
The Significance of Diffusion in Biological Systems
Diffusion is particularly crucial in biological systems, where it underpins many essential processes that sustain life:
- Cellular Transport: Diffusion is responsible for the movement of nutrients, gases, and waste products across cell membranes. This is essential for cell survival and function.
- Nerve Impulse Transmission: The transmission of nerve impulses involves the diffusion of ions across the nerve cell membrane.
- Muscle Contraction: Muscle contraction is triggered by the diffusion of calcium ions within muscle cells.
- Photosynthesis: In plants, carbon dioxide diffuses from the air into the leaves, where it is used for photosynthesis.
- Plant Nutrient Uptake: Plants absorb nutrients from the soil through diffusion.
Current Trends and Future Directions in Diffusion Research
The study of diffusion continues to be an active area of research, with ongoing efforts to understand its complexities and harness its potential in various applications. Some current trends and future directions include:
- Developing More Efficient Drug Delivery Systems: Researchers are working on developing drug delivery systems that can precisely control the rate and location of drug diffusion, improving the efficacy and safety of medications.
- Understanding Diffusion in Complex Biological Environments: Scientists are investigating how diffusion is affected by the complex microenvironment within cells and tissues, including the presence of crowding agents and other biomolecules.
- Using Diffusion for Nanotechnology Applications: Diffusion is being explored as a tool for fabricating nanoscale structures and devices.
- Modeling and Simulating Diffusion Processes: Computational models and simulations are being used to predict and understand diffusion behavior in various systems, from biological tissues to industrial processes.
Expert Tips for Understanding and Applying Diffusion Concepts
- Visualize the Process: Think of diffusion as a natural tendency for molecules to spread out and equalize concentrations.
- Understand Fick's Law: Familiarize yourself with Fick's First Law and how it relates diffusion flux to the concentration gradient and diffusion coefficient.
- Consider All Factors: Remember that temperature, molecular weight, medium density, and membrane permeability can all influence diffusion.
- Apply the Concepts: Look for examples of diffusion in your everyday life and in the natural world.
Frequently Asked Questions (FAQ)
-
Q: What is the difference between diffusion and osmosis?
- A: Diffusion is the movement of any molecule from an area of high concentration to an area of low concentration. Osmosis is a specific type of diffusion that involves the movement of water across a semipermeable membrane from an area of high water concentration (low solute concentration) to an area of low water concentration (high solute concentration).
-
Q: Does diffusion require energy?
- A: Simple and facilitated diffusion are passive processes, meaning they do not require the cell to expend energy. The movement of molecules is driven by the concentration gradient.
-
Q: What is meant by "net movement" in diffusion?
- A: While individual molecules move randomly in all directions, there is a statistically higher probability that molecules will move from an area of high concentration to an area of low concentration. This results in a "net movement" in that direction.
-
Q: Can diffusion occur in solids?
- A: Yes, diffusion can occur in solids, but it is typically much slower than in liquids or gases.
-
Q: How does diffusion relate to entropy?
- A: Diffusion increases entropy (disorder) in a system by distributing molecules more evenly.
Conclusion
Diffusion, driven by the relentless force of concentration gradients, is a fundamental process that underpins countless phenomena in our world. From the air we breathe to the intricate workings of our cells, diffusion plays a vital role in sustaining life and shaping our environment. By understanding the principles of diffusion and the factors that influence it, we can gain a deeper appreciation for the complexity and interconnectedness of the natural world. And, perhaps more importantly, we can use this knowledge to develop new technologies and solutions to address some of the world's most pressing challenges, from drug delivery to environmental remediation.
How will you apply your understanding of diffusion in your daily life or future endeavors? What innovations might be possible with a deeper understanding of this essential process?
Latest Posts
Latest Posts
-
Protons Neutrons And Electrons On Periodic Table
Nov 08, 2025
-
Examples For The Third Law Of Motion
Nov 08, 2025
-
Differentiate Between Physical Activity And Exercise
Nov 08, 2025
-
Inequalities With Variables On Both Sides
Nov 08, 2025
-
Electromagnetic Waves Are Longitudinal Or Transverse
Nov 08, 2025
Related Post
Thank you for visiting our website which covers about Diffusion Is A Process Which Depends On Concentration Gradients. . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.