1s 2 2s 2 2p 6 3s 2 3p 4
pythondeals
Nov 17, 2025 · 11 min read
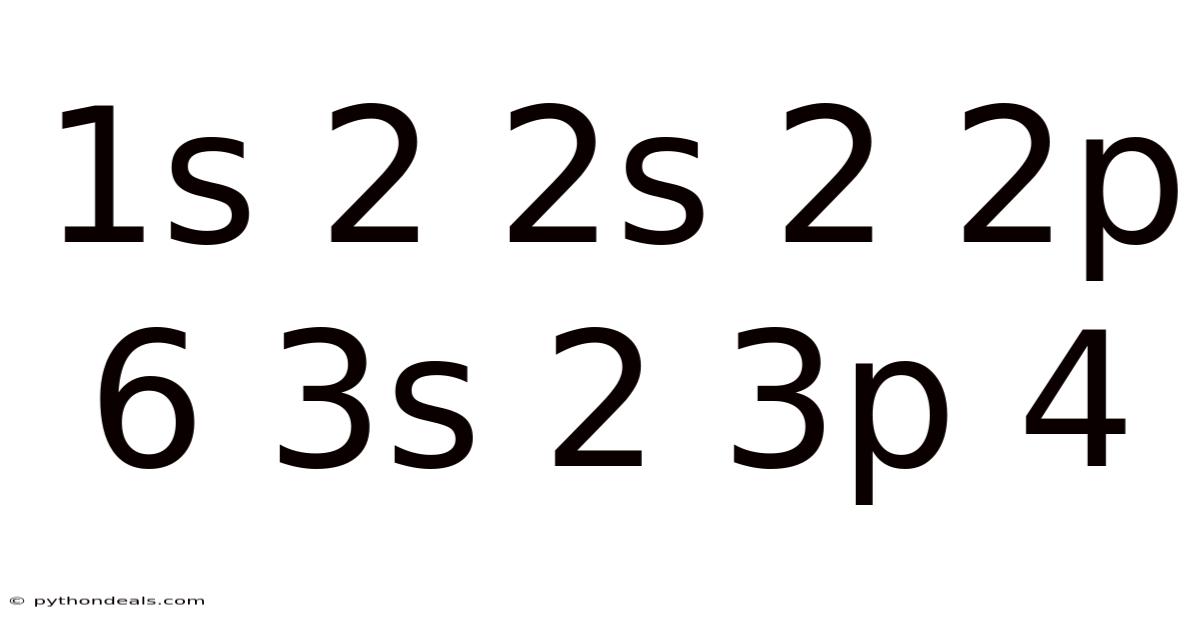
Table of Contents
Decoding the Electron Configuration: 1s² 2s² 2p⁶ 3s² 3p⁴
Have you ever looked at a seemingly random series of numbers and letters and wondered what it represented? In the realm of chemistry, these symbols often unveil the secrets of an atom's electron arrangement, a fundamental aspect dictating its properties and behavior. Specifically, the electron configuration 1s² 2s² 2p⁶ 3s² 3p⁴ paints a detailed picture of how electrons are distributed within an atom, influencing its reactivity and the types of bonds it forms. Understanding this notation unlocks a deeper comprehension of the periodic table and the elements that constitute our world.
Imagine an atom as a multi-story building, each floor representing a different energy level and each room (orbital) holding a maximum of two residents (electrons). The address of each resident is precisely defined by the electron configuration. This address reveals which floor (energy level), which room (orbital), and how many residents occupy that specific room. This detailed information enables us to predict how the atom will interact with other atoms, ultimately determining the characteristics of the compounds it forms. The configuration 1s² 2s² 2p⁶ 3s² 3p⁴, in particular, describes an element that is vital to life and industry: sulfur. Let's delve into the intricacies of this configuration and uncover its significance.
Understanding Electron Configuration: A Comprehensive Overview
Electron configuration is a shorthand notation that describes the arrangement of electrons within an atom's electron shells and subshells. It follows specific rules and principles rooted in quantum mechanics, allowing us to predict the chemical behavior of elements.
- Shells and Subshells: Electrons orbit the nucleus in specific energy levels called electron shells, designated by principal quantum numbers (n = 1, 2, 3, etc.). Each shell contains one or more subshells, denoted by letters s, p, d, and f. Each subshell has a specific shape and energy level.
- Orbitals: Each subshell consists of one or more orbitals, which are regions of space where an electron is most likely to be found. An s subshell has one orbital, a p subshell has three orbitals, a d subshell has five orbitals, and an f subshell has seven orbitals. Each orbital can hold a maximum of two electrons with opposite spins (Pauli Exclusion Principle).
- Notation: The electron configuration notation lists the occupied subshells in order of increasing energy, with the number of electrons in each subshell written as a superscript. For example, 1s² indicates that the s subshell in the first energy level (n=1) contains two electrons.
To write the electron configuration of an element, we follow the Aufbau principle (also known as the building-up principle), which states that electrons first fill the lowest energy levels available to them. We also adhere to Hund's rule, which states that electrons will individually occupy each orbital within a subshell before doubling up in any one orbital. This minimizes electron-electron repulsion and results in a more stable configuration.
The sequence of filling orbitals is usually: 1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p, 5s, 4d, 5p, 6s, 4f, 5d, 6p, 7s, 5f, 6d, 7p. This order can be remembered with the help of the diagonal rule.
The power of understanding electron configuration lies in its ability to explain the periodic trends observed in the periodic table. Elements in the same group (vertical column) have similar valence electron configurations (electrons in the outermost shell), which explains their similar chemical properties. For example, all elements in group 1 (alkali metals) have an ns¹ valence configuration and tend to lose one electron to form +1 ions. Similarly, all elements in group 17 (halogens) have an ns²np⁵ valence configuration and tend to gain one electron to form -1 ions.
Deconstructing 1s² 2s² 2p⁶ 3s² 3p⁴: The Case of Sulfur
The electron configuration 1s² 2s² 2p⁶ 3s² 3p⁴ represents the element sulfur (S). Let's break it down:
- 1s²: The first energy level (n=1) contains two electrons in the s subshell. This subshell can hold a maximum of two electrons, so it's completely filled.
- 2s²: The second energy level (n=2) contains two electrons in the s subshell. Again, this subshell is completely filled.
- 2p⁶: The second energy level (n=2) also contains six electrons in the p subshell. The p subshell consists of three orbitals, each capable of holding two electrons. Therefore, the 2p subshell is also completely filled.
- 3s²: The third energy level (n=3) contains two electrons in the s subshell, making it completely filled.
- 3p⁴: The third energy level (n=3) also contains four electrons in the p subshell. This is where things get interesting. Since the 3p subshell can hold up to six electrons, it is not completely filled. According to Hund's rule, the first three electrons will individually occupy each of the three p orbitals. The fourth electron will then pair up with one of the electrons already in an orbital. This leaves two unpaired electrons in the 3p subshell.
The presence of two unpaired electrons in the 3p subshell is crucial to understanding sulfur's chemical behavior. These unpaired electrons make sulfur relatively reactive and allow it to form two covalent bonds, as seen in hydrogen sulfide (H₂S). However, sulfur can also expand its octet (have more than eight electrons in its valence shell) and form more complex compounds, such as sulfur dioxide (SO₂) and sulfuric acid (H₂SO₄).
Sulfur: Properties, Reactions, and Importance
Sulfur is a yellow, nonmetallic element belonging to group 16 (chalcogens) of the periodic table. Its electron configuration 1s² 2s² 2p⁶ 3s² 3p⁴ gives rise to its characteristic properties and reactivity.
- Physical Properties: Sulfur exists as a solid at room temperature and has a relatively low melting point (115.21 °C) and boiling point (444.6 °C). It exists in various allotropic forms, meaning it can exist in different structural modifications. The most common form is cyclic S₈, where eight sulfur atoms are bonded together in a ring.
- Chemical Properties: Sulfur is relatively reactive and can combine directly with most elements, except for noble gases. Its reactivity is due to the presence of two unpaired electrons in its valence shell. Sulfur can act as both an oxidizing agent (accepting electrons) and a reducing agent (donating electrons), depending on the reaction conditions.
- Reactions:
- With Oxygen: Sulfur readily reacts with oxygen to form sulfur dioxide (SO₂), a colorless gas with a pungent odor. SO₂ is a major air pollutant and contributes to acid rain. Further oxidation of SO₂ can produce sulfur trioxide (SO₃), which reacts with water to form sulfuric acid (H₂SO₄).
- With Hydrogen: Sulfur reacts with hydrogen at elevated temperatures to form hydrogen sulfide (H₂S), a colorless gas with a characteristic rotten egg smell. H₂S is highly toxic and corrosive.
- With Metals: Sulfur reacts with many metals to form metal sulfides. For example, it reacts with iron to form iron sulfide (FeS), also known as pyrite or "fool's gold."
- Importance: Sulfur plays a crucial role in various industries and biological processes.
- Sulfuric Acid Production: The majority of sulfur produced is used to manufacture sulfuric acid (H₂SO₄), one of the most important industrial chemicals. Sulfuric acid is used in the production of fertilizers, detergents, plastics, and many other products.
- Vulcanization of Rubber: Sulfur is used to vulcanize rubber, a process that improves its strength, elasticity, and durability. Vulcanization involves cross-linking the polymer chains in rubber with sulfur atoms.
- Agriculture: Sulfur is an essential nutrient for plants and is often added to fertilizers. It is particularly important for the synthesis of proteins and enzymes.
- Biological Processes: Sulfur is a component of several important amino acids, such as cysteine and methionine, which are essential for protein structure and function. It is also a component of several vitamins and coenzymes.
Beyond the Basics: Advanced Concepts and Applications
While the electron configuration 1s² 2s² 2p⁶ 3s² 3p⁴ provides a fundamental understanding of sulfur's electronic structure, more advanced concepts can provide further insight into its behavior.
- Orbital Diagrams: Orbital diagrams are a visual representation of electron configuration, showing the individual orbitals within each subshell and the spin of each electron. For sulfur, the orbital diagram for the 3p subshell would show three orbitals, with one electron in each of the first two orbitals and two electrons in the third orbital, one with an up spin and one with a down spin.
- Spectroscopic Data: Spectroscopic data, such as photoelectron spectroscopy (PES), can provide experimental confirmation of the electron configuration. PES measures the binding energies of electrons in an atom and can be used to identify the occupied energy levels and their relative populations.
- Computational Chemistry: Computational chemistry methods, such as density functional theory (DFT), can be used to calculate the electronic structure of molecules containing sulfur and predict their properties. These methods provide a more accurate and detailed picture of the electron distribution and bonding in sulfur compounds.
- Reactivity Predictions: Understanding the electron configuration of sulfur allows us to predict its reactivity with other elements and compounds. For example, knowing that sulfur has two unpaired electrons and can expand its octet allows us to predict the formation of compounds such as SO₂, SO₃, SF₄, and SF₆.
Trends and Recent Developments
In recent years, research on sulfur and its compounds has focused on several key areas:
- Sulfur Polymers: Researchers are exploring the use of sulfur as a building block for new polymers. These sulfur polymers have potential applications in energy storage, environmental remediation, and biomedicine.
- Sulfur-Based Batteries: Sulfur is being investigated as a cathode material in lithium-sulfur batteries. These batteries have the potential to offer higher energy densities than traditional lithium-ion batteries.
- Sulfur in Environmental Remediation: Sulfur compounds are being used to remove heavy metals and other pollutants from contaminated water and soil.
- Sulfur in Drug Discovery: Sulfur-containing compounds are being investigated as potential drugs for a variety of diseases, including cancer, HIV, and malaria.
Expert Advice and Tips
- Master the Basics: Ensure you have a solid understanding of electron shells, subshells, and orbitals before delving into more complex concepts.
- Practice, Practice, Practice: Writing electron configurations requires practice. Work through numerous examples to become proficient.
- Use the Periodic Table as a Guide: The periodic table is an invaluable tool for predicting electron configurations. The group number indicates the number of valence electrons, and the period number indicates the highest occupied energy level.
- Understand Hund's Rule: Hund's rule is essential for correctly filling orbitals within a subshell. Remember that electrons will individually occupy each orbital before pairing up.
- Don't Be Afraid to Ask for Help: If you're struggling to understand electron configuration, don't hesitate to ask your teacher, professor, or a fellow student for help. There are also many online resources available to assist you.
Frequently Asked Questions (FAQ)
- Q: What is the difference between electron configuration and valence electron configuration?
- A: Electron configuration describes the arrangement of all electrons in an atom, while valence electron configuration describes only the electrons in the outermost shell (valence shell).
- Q: How can I determine the number of valence electrons from the electron configuration?
- A: The number of valence electrons is equal to the sum of the superscripts for the s and p subshells in the highest occupied energy level. For sulfur (1s² 2s² 2p⁶ 3s² 3p⁴), the highest occupied energy level is n=3, and there are two electrons in the 3s subshell and four electrons in the 3p subshell, for a total of six valence electrons.
- Q: What are the exceptions to the Aufbau principle?
- A: There are some exceptions to the Aufbau principle, particularly for transition metals. For example, chromium (Cr) has the electron configuration [Ar] 3d⁵ 4s¹, not [Ar] 3d⁴ 4s², because a half-filled d subshell is more stable.
- Q: How does electron configuration relate to ionization energy?
- A: Ionization energy is the energy required to remove an electron from an atom. Elements with stable electron configurations (e.g., noble gases) have high ionization energies, while elements with less stable configurations (e.g., alkali metals) have low ionization energies.
Conclusion
The electron configuration 1s² 2s² 2p⁶ 3s² 3p⁴ is more than just a series of numbers and letters. It is a key to understanding the properties and behavior of sulfur, a vital element with applications spanning from industrial processes to biological systems. By understanding the principles of electron configuration and applying them to specific elements, we can unlock a deeper appreciation for the intricate workings of the chemical world. This knowledge empowers us to predict chemical reactions, design new materials, and develop innovative technologies.
How will you use this knowledge to further explore the fascinating world of chemistry? Are you intrigued to investigate other elements and their electron configurations? The journey into the realm of electron configuration is an ongoing exploration, and the discoveries are limitless.
Latest Posts
Latest Posts
-
How Many Sides Are In A Regular Polygon
Nov 17, 2025
-
Find Line Of Intersection Of Two Planes
Nov 17, 2025
-
Sum Of Terms In Arithmetic Sequence
Nov 17, 2025
-
Earth God Of Woods And Fields Half Man Half Goat
Nov 17, 2025
-
A Liquid Substance Capable Of Dissolving Other Substances
Nov 17, 2025
Related Post
Thank you for visiting our website which covers about 1s 2 2s 2 2p 6 3s 2 3p 4 . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.